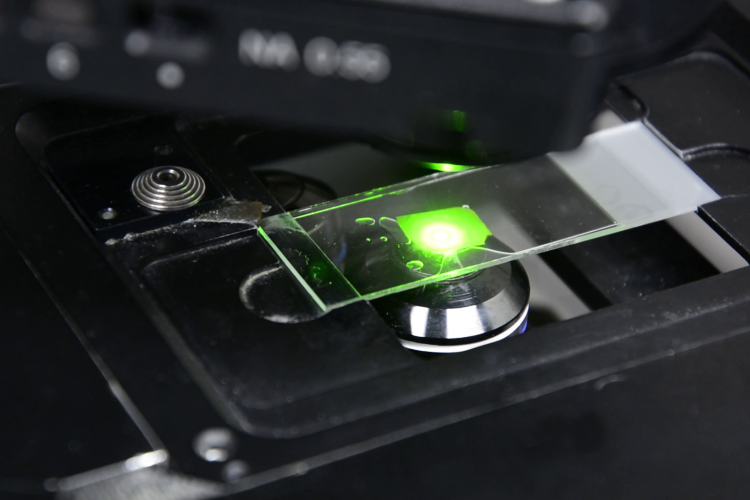
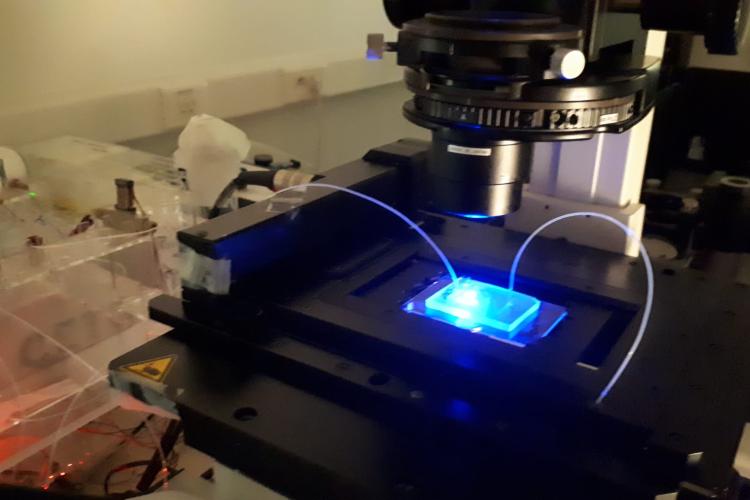
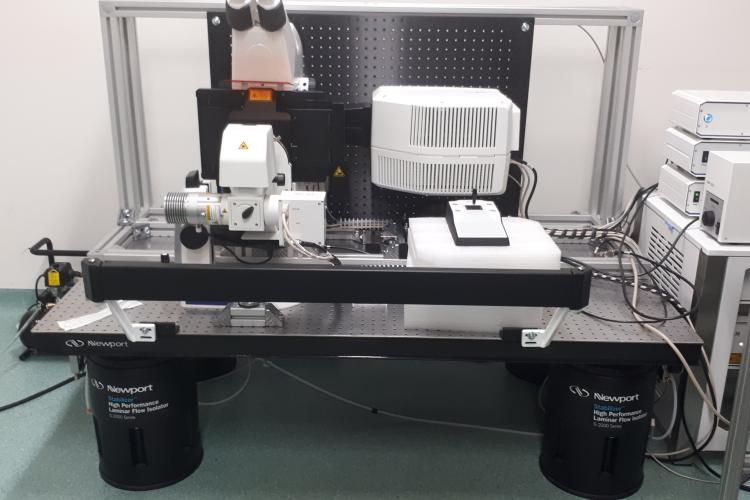
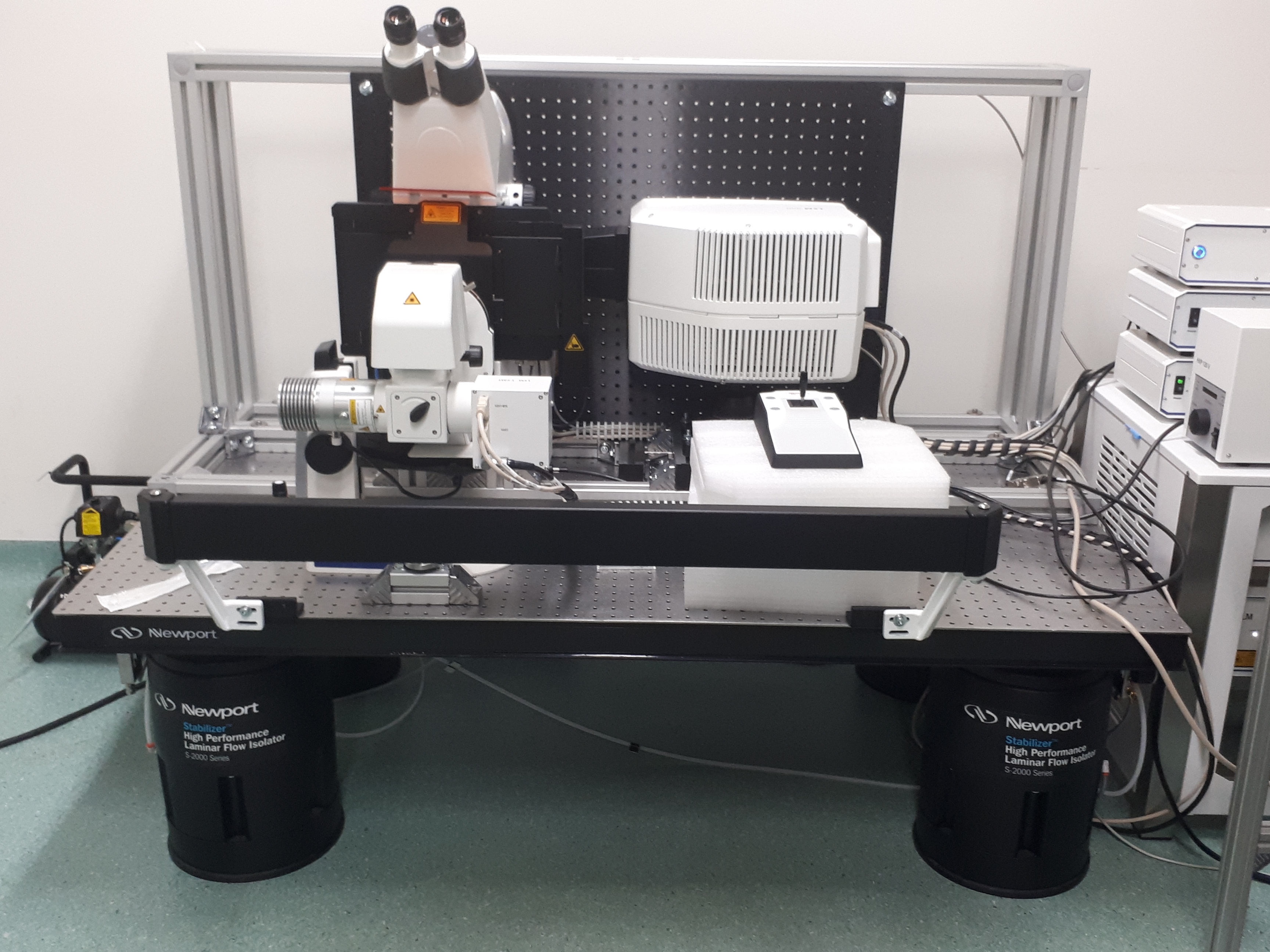
To support live cell imaging of diverse plant tissues, PSB maintains a diverse set of microscopes that are freely available to all researchers working in the center.
Imaging capacities at PSB range from standard light microscopy, epifluorescence high-content systems to spinning disk imaging, point-scanning confocal and fluorescence life time imaging.
PSB researchers are also actively involved in generating tools to allow real-time visualization of plants under conditions that disturb specific processes. Examples are the use of microfluidics setups to monitor the effect of chemical compounds as well as the effect of altered temperature, on cellular dynamics.
For advice and guidance with your imaging experiment, please contact Daniel Van Damme ([email protected]) and Evelien Mylle ([email protected]).
Microscopy Infrastructure
Overview of all fluorescence and SEM microscopes in the department
Visit the PSB intranet for detailed information about the microscopes (PSB members only). https://intranet.psb.ugent.be
Point-scanning confocals
LSM900 inverted
The unique feature of this microscope is its ability to track a plant root over a long period of time during its growth along the gravity vector. This is achieved using a root tracking software module, combined with a vertical point scanning microscope setup.
FV1000 Olympus
The Fluoview software has a very user-friendly interface for imaging multi area time lapses with individual image settings for each selected point.
This system is equipped with a time domain FLIM module (Picoquant) for ‘Fluorescence Lifetime Imaging Microscopy’ with a pulsed diode 440 nm laser, and a GFP emission filter.
SP8X Leica

The uniqueness of this inverted confocal microsope is the wide range of excitation laser lines that can be used (continuous WhiteLight laser), combined with the option of capturing the lifetime component of the emission light on the Hyd detectors. This allows to remove unwanted signal based on lifetime using LightGate, ideal for eliminating autofluorescence and reflection. With the very accurate SMD Hyd, lifetime differences can be very easily and quickly visualized (FALCON, Fast Lifetime CONtrast).
LSM 710 Zeiss
This inverted confocal microscope has 405nm/458nm/488nm/559nm/633nm laser excitation lines. There is an adjustable spectral emission window on two detectors. An extra slit in front of the UV emission can be inserted in the microscope allowing fast photoconversion of photoswitchable fluorophores on a small spot or a line.
Spinning disc confocals
Ultraview Perkin Elmer
When speed is of superior demand rather than resolution, this system is the most convenient choice. Ratiometric imaging (Ca2+ sensors, phosphorylation sensors), 4D stitching and photokinesis FRAP on fly are examples of the possibilities that this system offers.
Epifluorescence microscopes
Axiovert 135M Zeiss
This is an inverted microscope with automated stage and shutters controlled by the Axiovision software, ideal for generating multi area time lapses and high content screening purposes. Filter sets are available for DAPI/CFP/GFP/YFP/RFP/GFP-RFP/CFP-YFP. Several objective lenses, up to 40x dry, are available. Available lenses include 10x, 20x dry, 40x dry and 40x oil corrected.
Axioimager M1 Zeiss
This is an upright microscope with manual stage controlled by the Axiovision software and Zen blue. Filter sets are available for DAPI/GFP/RFP/CFP-YFP/CFP-YFP-FRET. Several objective lenses up to 40x dry are available and also water dipping lenses (20x/40x/63x) can fitted onto this microscope for screening purposes. Available lenses include 10x, 20x dry, 40x dry and 40x oil corrected.
Macroscope MVX10 Olympus
<INSERT PICTURE 10>
This microscope has been tilted horizontally to allow low magnification visualization of fluorescent markers during gravistimulation experiments. It has automated shutters, although it is still equipped with a manual stage. This system allows to follow root growth during several (up to 40) hours, allowing to correlate fluorescent marker expression with root development and growth. Filter sets are available for GFP, YFP and RFP. Available lenses include 1x and 2x.
Stereomicroscopes MZ16F (Leica GFP2) Leica and Meica M165FC (Leica GFP3)
<INSERT PICTURE 12>
<INSERT PICTURE 13>
These microscopes have filter sets for TagBFP2, GFP and RFP and are equipped with a Leica DFZ7000 T camera. These microscopes mainly serve screening purposes and fluorescence-based selection of transformed seeds (FAST selection).
Olympus ScanR
<INSERT PICTURE 14>
This inverted microscope is a high content screening platform, equipped with an automated stage, automated multi-channel imaging and autofocus. The software allows automated image analysis. Ideal for high throughput 96-well plate imaging.
TM-1000 Hitachi
<INSERT PICTURE 15>
This tabletop SEM machine is user friendly and allows detailed observation of samples, which are resistant against low vacuum conditions (e.g. pollen grains or trichomes). It is also widely used to scan nail-polish replica samples of dental-resin impressions of leave tissue to follow leaf growth over time (several days with one impression of a growing leaf each day).
CherryTemp Heater Cooler for microscopy
<INSERT PICTURE 16>
The CherryTemp system (Cherrybiotech) allows temperature shifts of a sample within 10 seconds between 5 °C and 45°C. The system can be easily mounted on our Spinning Disc and Zeiss 710 confocal. The use of the CherryTemp system on Arabidopsis seedlings is demonstrated in the movie: https://www.cherrybiotech.com/heater-cooler-for-microscopy/temperature-control-for-plant-microscopy.
Microfluidic setup - IN PREPARATION
<INSERT PICTURE 18>
A microfluidic setup is in preparation in order to monitor rapid manipulation of root environment (Grossmann et al. Jove, 2012)