Temperature-modulated live cell imaging.
Dynamic imaging of fast-occurring processes is hampered by the fact that the signal/noise ratio of a fluorescent image is inversely related to the exposure time. The exposure time in turn limits the imaging speed and thereby the temporal resolution. We are increasing the temporal resolution of live-cell imaging plant samples by modulating the temperature of the samples in situ on the microscope stage as lowering the temperature slows down biological processes.
CME is a fast and stepwise process and involves the sequential recruitment of several players. Adaptor complexes, the clathrin scaffold and endocytic accessory proteins all arrive and depart at different time points during the process. To visualize the temporal dynamics of the various players, we are generating fluorescently tagged functional fusions of TPC subunits and are performing dual color spinning disc microscopy on complemented double mutant lines at ambient and at lowered temperature. Imaging endocytosis at lowered temperature delays the process and allowed us to increase the temporal resolution by which the effector proteins are recruited to the plasma membrane.
Next to this, we use our temperature-modulatory setup also to study the effect of high temperature stress on plants. Here we combine live-cell imaging of fluorescently tagged proteins with temperature changes to observe how this alters their localization and properties.
Wang et al., Plant Physiology 2020 PMID: 32321842
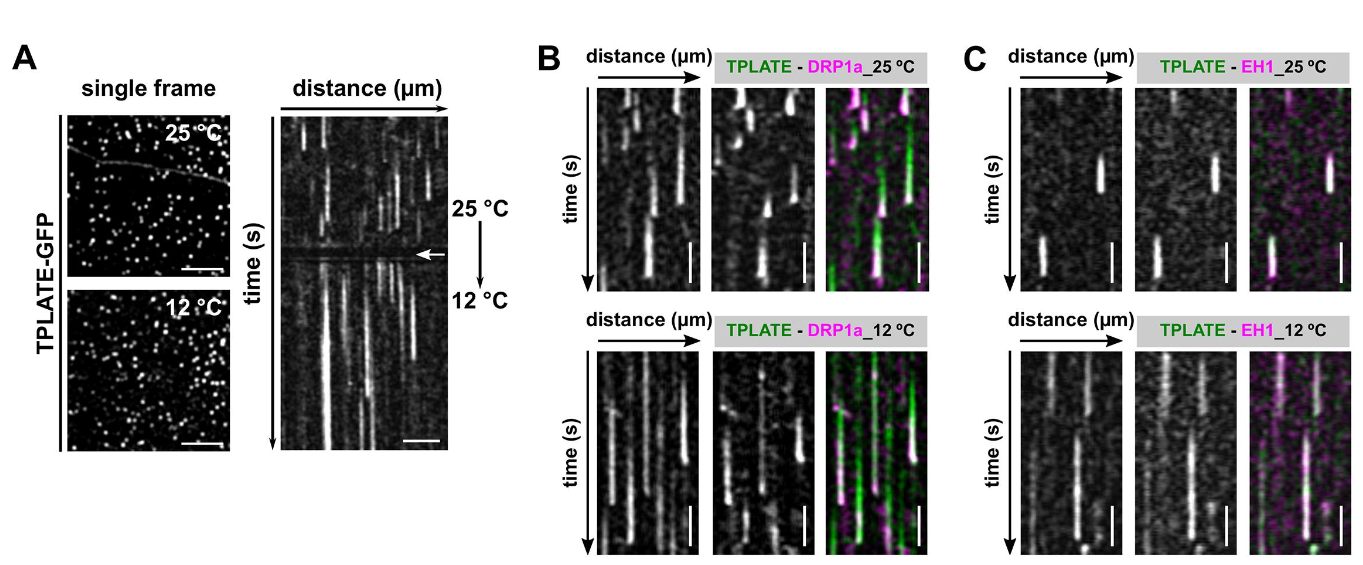
Temperature-modulated imaging in Arabidopsis. Panel A shows endocytic foci in etiolated hypocotyl cells expressing TPLATE-GFP and the corresponding kymographs as the cells transition from 25 degrees to 10 degrees. The length of the traces, proxy for the duration of endocytosis, increases with lowering the temperature. Panels B and C show the effect of lowering the temperature of two combinations of endocytic markers, TPLATE and DRP1a and TPLATE and EH1. Image adapted from Wang et al., 2020.