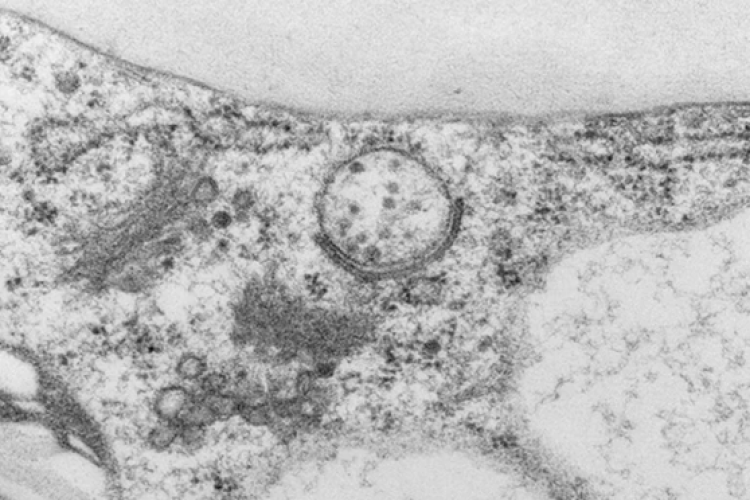
Autophagy, derived from Greek “self-eating”, is an important process for recycling of cellular material. Autophagy is initiated in response to environmental stresses such as nutrient starvation, and acts to re-mobilise nutrients which enhances stress tolerance and promotes plant survival. This pathway involves the formation of a double membrane structure termed the ‘autophagosome’, which fuses with the vacuole resulting in the degradation of cellular material.
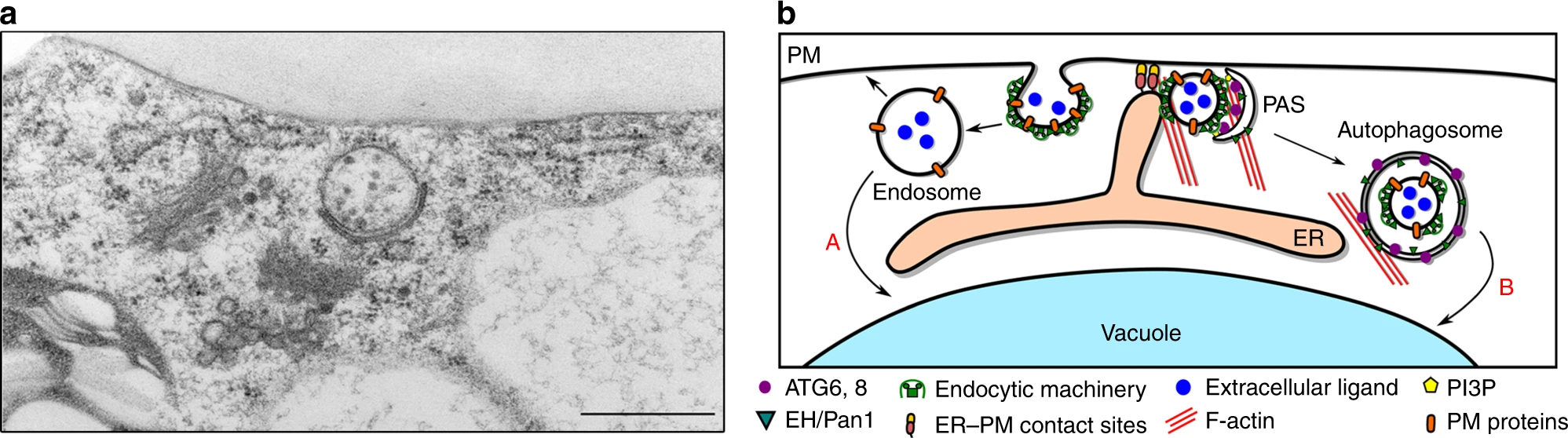
In the Van Damme laboratory, we recently identified a novel link between the endocytosis and autophagy pathways (Wang and Pleskot et al., 2019). We found that two subunits of the TPLATE complex (TPC), AtEH1 and AtEH2 are involved in autophagy at the plasma membrane. AtEH1/2 interact with the actin activator ARP2/3, and the protein VAP27-1, an important component of endoplasmic reticulum-plasma membrane contact sites (Fig. 1). This autophagy pathway is implicated in the degradation of the TPLATE complex and plasma membrane components.
Current work in the lab is focused on identifying the specific plasma membrane cargo proteins which are degraded through this endo-autophagy pathway. Furthermore, we are analysing whether specific stress conditions induce this degradation pathway.
References:
Wang, P. et al. Plant AtEH/Pan1 proteins drive autophagosome formation at ER-PM contact sites with actin and endocytic machinery. Nat. Commun. 10, 1–16 (2019).